Question:
Identify the correct molecular picture showing what happens at the critical micellar concentration (CMC) of an aqueous solution of a surfactant ( polar head,
polar head,  non-polar tail,
non-polar tail,  water $)$.
water $)$.
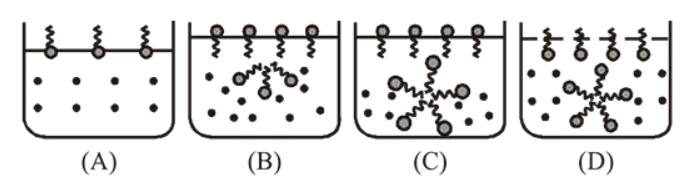
Correct Option:
Solution:
(a) In micelle formation, above "CMC" hydrocarbon chains are pointing towards the centre of sphere with $\mathrm{COO}^{-}$part remaining outward on the surface.
