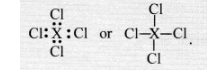Question:
If an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride ?
Solution:
The element X present in group 14 has four valence electrons in its atom. It can complete its octet by sharing four valence electrons with the
electrons of other atoms. Therefore, it will form covalent bonds with the four atoms of chlorine. The formula of the chloride of the element X is.