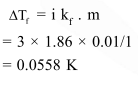Question:
If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the change in freezing point of water $\left(\Delta \mathrm{T}_{\mathrm{f}}\right)$, when $0.01 \mathrm{~mol}$ of sodium sulphate isdissolved in $1 \mathrm{~kg}$ of water, is $\left(\mathrm{K}_{\mathrm{f}}=1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\right)$ :-
Correct Option: , 3
Solution: