Question:
In a molecule of pyrophosphoric acid, the number of $\mathrm{P}-\mathrm{OH}, \mathrm{P}=\mathrm{O}$ and $\mathrm{P}-\mathrm{O}-\mathrm{P}$ bonds/ moiety(ies) respectivey are :
Correct Option: , 4
Solution:
Pyrophosphoric acid.
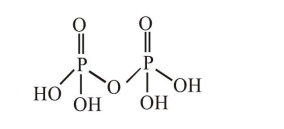
$\mathrm{P}-\mathrm{OH}$ linkages $=4$
$P=O$ linkages $=2$
$\mathrm{P}-\mathrm{O}-\mathrm{P}$ linkages $=1$
