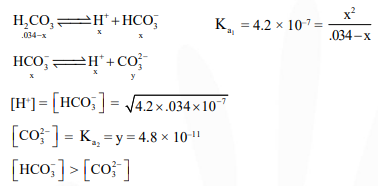Question:
In aqueous solution the ionization constants for carbonic acid are $\mathrm{K}_{1}=4.2 \times 10^{-7}$ and $\mathrm{K}_{2}=4.8$ $\times 10^{-11}$ Select the correct statement for a saturated $0.034 \mathrm{M}$ solution of the carbonic acid :-
Correct Option: , 3
Solution: