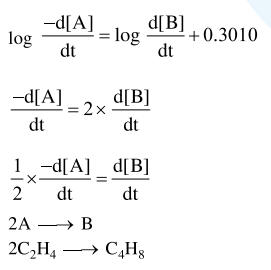Question:
In the following reaction; $\mathrm{xA} \rightarrow \mathrm{yB}$
$\log _{10}\left[-\frac{\mathrm{d}[\mathrm{A}]}{\mathrm{dt}}\right]=\log _{10}\left[\frac{\mathrm{d}[\mathrm{B}]}{\mathrm{dt}}\right]+0.3010$
'A' and 'B' respectively can be :
Correct Option: , 2
Solution: