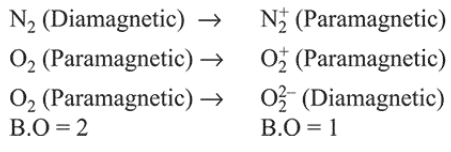Question:
In which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic?
Correct Option:
Solution:
In case of $\mathrm{NO}$ (paramagnetic) $\rightarrow \mathrm{NO}^{+}$(diamagnetic) the bond order has increased from $2.5$ to 3 .
For other cases: