Question:
Increasing order of reactivity of the following compounds for $S_{N} 1$ substitution is:
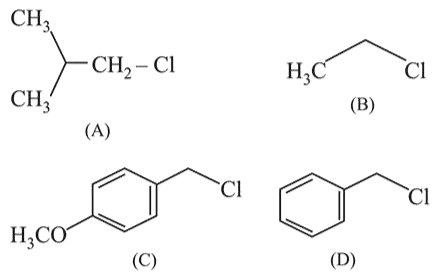
Correct Option: , 3
Solution:
In $\mathrm{S}_{\mathrm{N}} 1$ reaction carbocation acts as an intermediate.
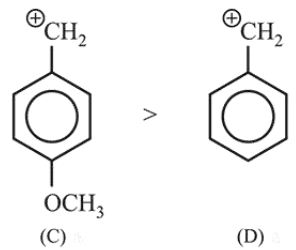
Carbocation produced by $(\mathrm{C})$ is more stable than carbocation produced by (D) due to + I effect of $-\mathrm{OCH}_{3}$ group.
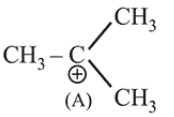
Further in (A) there is formation of tertiary carbocation after rearrangement while (B) is primary carbocation.
So, the correct order is $(C)>(D)>(A)>(B)$.
