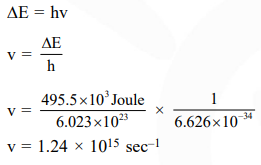
Question:
Ionization energy of gaseous $\mathrm{Na}$ atoms is $495.5 \mathrm{kjmol}^{-1}$. The lowest possible frequency of light that ionizes a sodium atom is $\left(\mathrm{h}=6.626 \times 10^{-34} \mathrm{Js}, \mathrm{N}_{\mathrm{A}}=6.022 \times 10^{23} \mathrm{~mol}^{-1}\right)$
Correct Option: , 3
Solution: