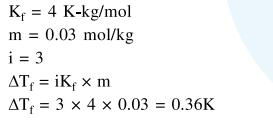Question:
Molal depression constant for a solvent is $4.0 \mathrm{~kg} \mathrm{~mol}^{-1}$. The depression in the freezing point of the solvent for $0.03 \mathrm{~mol} \mathrm{} \mathrm{kg}^{-1}$ solution of $\mathrm{K}_{2} \mathrm{SO}_{4}$ is :
(Assume complete dissociation of the electrolyte)
Correct Option: , 2
Solution: