Question:
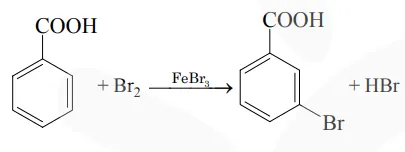
Consider the above reaction where $6.1 \mathrm{~g}$ of benzoic acid is used to get $7.8 \mathrm{~g}$ of m-bromo benzoic acid. The percentage yield of the product is_________.
(Round off to the Nearest integer)
[Given : Atomic masses : $\mathrm{C}=12.0 \mathrm{u}, \mathrm{H}: 1.0 \mathrm{u}$, $\mathrm{O}: 16.0 \mathrm{u}, \mathrm{Br}=80.0 \mathrm{u}]$
Solution:
Moles of Benzoic acid $=\frac{6.1}{122}$
$=$ moles of m-bromobenzoic acid
So, weight of m-bromobenzoic acid
$=\frac{6.1}{122} \times 201 \mathrm{gm}$
$=10.05 \mathrm{gm}$
$\%$ yield $=\frac{\text { Actual weight }}{\text { Theoretical weight }} \times 100$
$=\frac{7.8}{10.05} \times 100$
$=77.61 \%$
