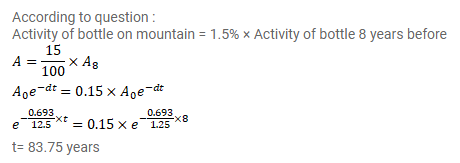Question:
Natural water contains a small amount of tritium (13H). This isotope beta-decay with a half-life of $12.5$ years. A mountaineer while climbing towards a difficult peak finds debris of some earlier unsuccessful attempt. Among other things he finds a sealed bottle of whisky. On return he analyses the whisky and finds that it contains only $1.5$ percent of the $13 \mathrm{H}$ radioactive as compared to a recently purchased bottle marked ' 8 years old'. Estimate the time of that unsuccessful attempt.
Solution: