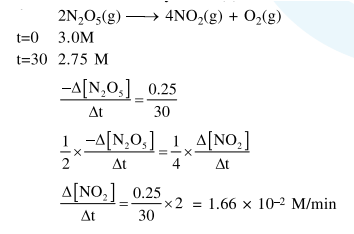Question:
$\mathrm{NO}_{2}$ required for a reaction is produced by the decomposition of $\mathrm{N}_{2} \mathrm{O}_{5}$ in $\mathrm{CCl}_{4}$ as per the equation
$2 \mathrm{~N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \rightarrow 4 \mathrm{NO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g})$
The initial concentration of $\mathrm{N}_{2} \mathrm{O}_{5}$ is $3.00 \mathrm{~mol} \mathrm{~L}^{-1}$ and it is $2.75 \mathrm{~mol} \mathrm{~L}^{-1}$ after 30 minutes. The rate of formation of $\mathrm{NO}_{2}$ is :
Correct Option: , 4
Solution: