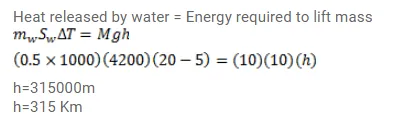Question:
On a winter day the temperature of the tap water is $20^{\circ} \mathrm{C}$ whereas the room temperature is $5^{\circ} \mathrm{C}$. Water is stored in a tank of capacity $0.5 \mathrm{~m}^{3}$ for household use. If it were possible to use the heat liberated by the water to lift a $10 \mathrm{~kg}$ of mass vertically, how high can it be lifted as the water comes to the room temperature? Take $\mathrm{g}=10 \mathrm{~m} / \mathrm{s}^{2}$.
Solution: