Question:
On heating compound (A) gives a gas (B) which is a constituent of air. This gas when treated with $\mathrm{H}_{2}$ in the presence of a catalyst gives another gas (C) which is basic in nature. (A) should not be :
Correct Option: , 3
Solution:
$\mathrm{Cu}^{2+}$ ions get precipitated every quickely due to low $K_{\mathrm{sp}}$ value even at very low concentration of $\mathrm{S}^{2-}$ ion.
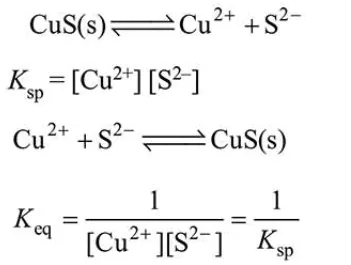
Due to high value of $K_{\text {eq }}$, CuS precipitated easily.
