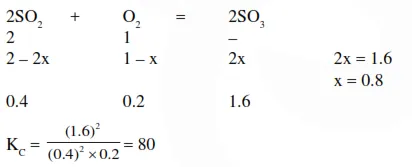Question:
One mole of $\mathrm{O}_{2}(\mathrm{~g})$ and two moles of $\mathrm{SO}_{2}(\mathrm{~g})$ were heated in a closed vessel of one litre capacity at $1098 \mathrm{~K}$. At equilibrium $1.6$ moles of $\mathrm{SO}_{3}(\mathrm{~g})$ were found. The equilibrium constant $\mathrm{K}_{\mathrm{C}}$ of the reaction would be :-
Correct Option: , 2
Solution: