Question:
One mole of an ideal gas at $27^{\circ} \mathrm{C}$ is taken from $\mathrm{A}$ to $\mathrm{B}$ as shown in the given $\mathrm{PV}$ indicator diagram. The work done by the system will be________ $\times 10^{-1} \mathrm{~J}$.
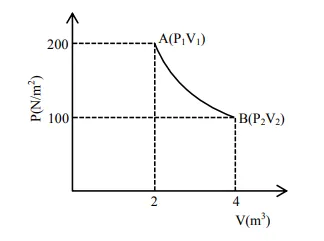
Solution:
Process of isothermal
$\mathrm{W}=\mathrm{nRT} \ell \mathrm{n}\left(\frac{\mathrm{V}_{2}}{\mathrm{~V}_{1}}\right)$
$=1 \times 8.3 \times 300 \times \ln 2$
$=17258 \times 10^{-1} \mathrm{~J}$
