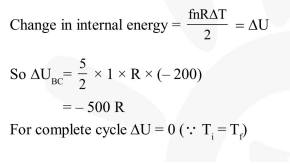
Question:
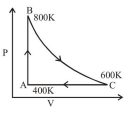
One mole of diatomic ideal gas undergoes a cyclic process $\mathrm{ABC}$ as shown in figure. The process $\mathrm{BC}$ is adiabatic. The temperatures at $\mathrm{A}, \mathrm{B}$ and $\mathrm{C}$ are $400 \mathrm{~K}, 800 \mathrm{~K}$ and $600 \mathrm{~K}$ respectively. Choose the correct statement :
Correct Option: , 2
Solution: