$5.1 \mathrm{~g} \mathrm{NH}_{4} \mathrm{SH}$ is introduced in $3.0 \mathrm{~L}$ evacuated flask at $327^{\circ} \mathrm{C}, 30 \%$ of the solid $\mathrm{NH}_{4} \mathrm{SH}$ decomposed to $\mathrm{NH}_{3}$ and $\mathrm{H}_{2} \mathrm{~S}$ as gases. The $K_{\mathrm{p}}$ of the reaction at $327^{\circ} \mathrm{C}$ is $(\mathrm{R}=0.082$ $\mathrm{L} \mathrm{atm} \mathrm{mol}^{-1} \mathrm{~K}^{-1}$, molar mass of $\mathrm{S}=32 \mathrm{~g} \mathrm{~mol}^{-1}$, molar mass of $\mathrm{N}=14 \mathrm{~g} \mathrm{~mol}^{-1}$ )
Correct Option: , 4
Concerned reaction:
$\mathrm{NH}_{4} \mathrm{SH} \longrightarrow \mathrm{NH}_{3}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{~S}(\mathrm{~g})$
Initial moles $=\frac{5.1}{51}=0.1 \mathrm{~mol}$
Moles at equillibrium
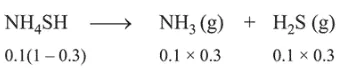
$\therefore \quad K_{c}=\left[\mathrm{NH}_{3}\right]\left[\mathrm{H}_{2} \mathrm{~S}\right]=\left(\frac{0.03}{3}\right)^{2}=10^{-4}$
$K_{p}=K_{c}(R T)^{\Delta n_{g}}$
$=10^{-4} \times(0.082 \times 600)^{2}=0.242 \mathrm{~atm}^{2}$
