Question:
At $1990 \mathrm{~K}$ and 1 atrm pressure, there are equal number of $\mathrm{Cl}_{2}$ molecules and $\mathrm{Cl}$ atoms in the reaction mixture. The value of $\mathrm{K}_{\mathrm{p}}$ for the reaction $\mathrm{Cl}_{2(\mathrm{~g})}=2 \mathrm{Cl}_{(\mathrm{g})}$ under the above conditions is $\mathrm{x} \times 10^{-1}$. The value of $x$ is ____________________. (Rounded off to the nearest integer)
Solution:
(5)
$\mathrm{Cl}_{2} \rightleftharpoons 2 \mathrm{Cl}$
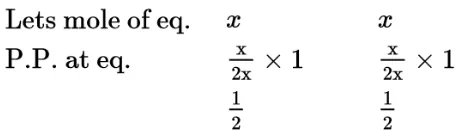
$\mathrm{K}_{\mathrm{p}}=\frac{\left[\mathrm{P}_{\mathrm{cl}}\right]^{2}}{\left[\mathrm{P}_{\mathrm{C}_{2}}\right]}=\frac{\left[\frac{1}{2}\right]^{2}}{\frac{1}{2}}=\frac{1}{2}=0.5=5 \times 10^{-1}$
$X=5$
