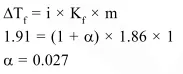Question:
The freezing point of a $1.00 \mathrm{~m}$ aqueous solution of $\mathrm{HF}$ is found to be $-1.91^{\circ} \mathrm{C}$. The freezing point constant of water, $\mathrm{K}_{\mathrm{f}}$, is $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$. The percentage dissociation of HF at this concentration is
Correct Option: 1
Solution: