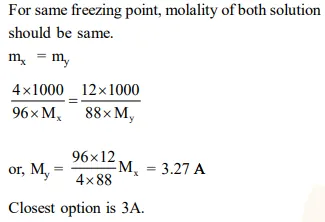
Question: Freezing point of a $4 \%$ aqueous solution of $\mathrm{X}$ is equal to freezing point of $12 \%$ aqueous solution of Y. If molecular weight of $\mathrm{X}$ is $\mathrm{A}$, then molecular weight of $Y$ is :-
$\mathrm{A}$
$3 \mathrm{~A}$
$4 \mathrm{~A}$
$2 \mathrm{~A}$
Correct Option: , 2
Solution: