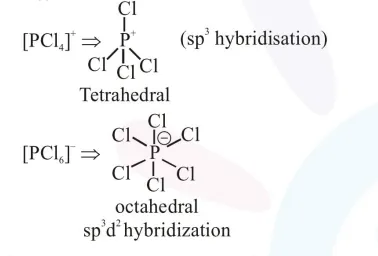
Question: The structure of $\mathrm{PCl}_{5}$ in the solid state is
square pyramidal
tetrahedral $\left[\mathrm{PCl}_{4}\right]^{+}$and octahedral $\left[\mathrm{PCl}_{6}\right]^{-}$
square planar $\left[\mathrm{PCl}_{4}\right]^{+}$and octahedral $\left[\mathrm{PCl}_{6}\right]^{-}$
trigonal bipyramidal
Correct Option: , 2
Solution:
$\mathrm{PCl}_{5(\mathrm{~s})}$ exist as $\left[\mathrm{PCl}_{4}\right]^{+}$and $\left[\mathrm{PCl}_{6}\right]^{-}$