Question:
Considering that $\Delta_{0}>\mathrm{P}$, the magnetic moment (in BM) of $\left[\mathrm{Ru}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ would be
Solution:
Magnetic moment (in B.M.) of $\left[\mathrm{Ru}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ would be; while considering that $\Delta_{0}>P$,
$\mathrm{Ru}_{(44)} ;[\mathrm{Kr}] 4 \mathrm{~d}^{7} 5 \mathrm{~s}^{1} \quad$ (in ground state)
$\Rightarrow$ In $\mathrm{Ru}^{2+} \Rightarrow 4 \mathrm{~d}^{6} \Rightarrow\left(\mathrm{t}_{2} \mathrm{~g}\right)^{6}(\mathrm{eg})^{0}$
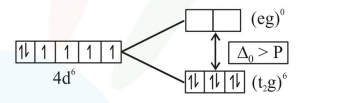
$\Rightarrow$ Here number of unpaired electrons in
$\mathrm{Ru}^{2+}=\left(\mathrm{t}_{2} \mathrm{~g}\right)^{6}(\mathrm{eg})^{0}=0$ and Hence
$\mu_{\mathrm{m}}=\sqrt{\mathrm{n}(\mathrm{n}+2)}$ B.M. $=0$ B.M.
