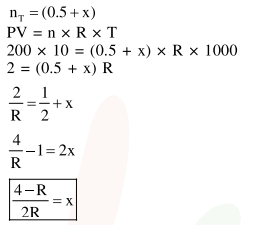Question:
$0.5$ moles of gas A and $x$ moles of gas B exert a pressure of $200 \mathrm{~Pa}$ in a a container of volume $10 \mathrm{~m}^{3}$ at $1000 \mathrm{~K}$. given $\mathrm{R}$ is the gas constant in $\mathrm{JK}^{-1} \mathrm{~mol}^{-1} \mathrm{~m}, \mathrm{x}$ is :
Correct Option: , 3
Solution: