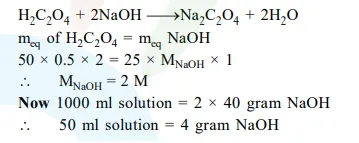Question: $50 \mathrm{~mL}$ of $0.5 \mathrm{M}$ oxalic acid is needed to neutralize $25 \mathrm{~mL}$ of sodium hydroxide solution. The amount of $\mathrm{NaOH}$ in $50 \mathrm{~mL}$ of the given sodium hydroxide solution is :
$40 \mathrm{~g}$
$20 \mathrm{~g}$
$80 \mathrm{~g}$
$10 \mathrm{~g}$
Correct Option:
Solution: