Question:
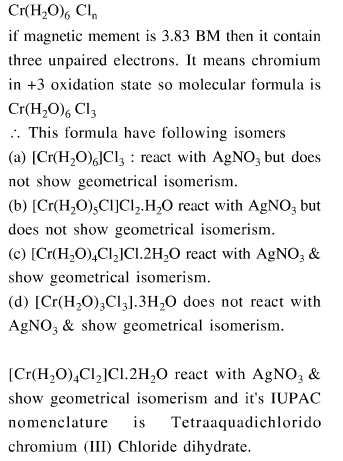
Complex $\mathrm{X}$ of composition $\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6} \mathrm{Cl}_{n}$ has a spin only magnetic moment of $3.83$ BM. It reacts with $\mathrm{AgNO}_{3}$ and shows geometrical isomerism. The IUPAC nomenclature of $X$ is :
Correct Option: 1
Solution: