Question:
The activity R of an unknown radioactive nuclide is measured at hourly intervals. The results found are tabulated as follows:
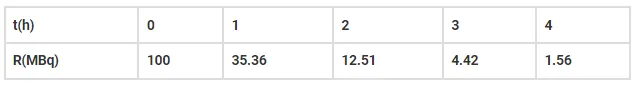
(i) Plot the graph of R versus t and calculate half-life from the graph.
(ii) Plot the graph of ln(R/R0) versus t and obtain the value of half-life from the graph.
Solution:
(i)
R(MBq)
t(h)
From graph we can say that the activity of R has reduced by 50%. Therefore, the half-life is 40 mins.
(ii) ln(R/Ro)
n(h)
Slope of the graph = – λ
λ = 1.05 h-1
Half-time = 0.693/ λ = 0.66 h = 39.6 min = 40 min
