Question: The correct statement about $\mathrm{ICl}_{5}$ and $\mathrm{ICl}_{4}^{-}$is :
both are is isostructural.
$\mathrm{ICl}_{5}$ is trigonal bipyramidal and $\mathrm{ICl}_{4}^{-}$is tetrahedral,
$\mathrm{ICl}_{5}$ is square pyramidal and $\mathrm{ICl}_{4}^{-}$is tetrahedral.
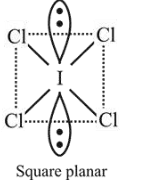
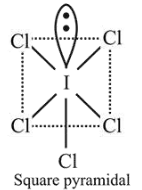
$\mathrm{ICl}_{5}$ is square pyramidal and $\mathrm{ICl}_{4}^{-}$is square planar.
Correct Option: , 4
Solution:
$\mathrm{ICl}_{5}$ is $s p^{3} d^{2}$ hybridised $(5 b p, 1 l p)$
$\mathrm{ICl}_{4}^{-}$is $s p^{3} d^{2}$ hybridised $(4 b p, 2 l p)$