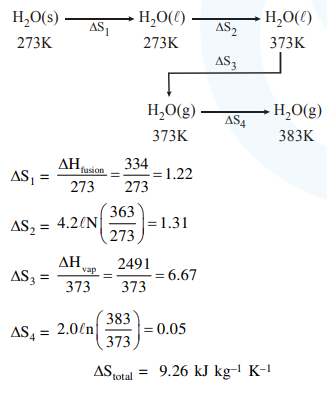
Question:
The entropy change associated with the conversion of $1 \mathrm{~kg}$ of ice at $273 \mathrm{~K}$ to water vapours at $383 \mathrm{~K}$ is :
(Specific heat of water liquid and water vapour are $4.2 \mathrm{~kJ} \mathrm{~K}^{-1} \mathrm{~kg}^{-1}$ and $2.0 \mathrm{~kJ} \mathrm{~K}^{-1} \mathrm{~kg}^{-1}$; heat of liquid fusion and vapourisation of water are $344 \mathrm{~kJ} \mathrm{~kg}^{-1}$ and $2491 \mathrm{~kJ} \mathrm{~kg}^{-1}$, respectively).
$(\log 273=2.436, \log 373=2.572, \log 383=2.583$ )
Correct Option: , 4
Solution: