Question:
The equilibrium constant $\left(\mathrm{K}_{\mathrm{C}}\right)$ for the reaction $\mathrm{N}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}(\mathrm{g})$ at temperature $\mathrm{T}$ is $4 \times 10^{-4}$. The value of $\mathrm{K}_{\mathrm{C}}$ for the reaction. $\mathrm{NO}(\mathrm{g}) \longrightarrow 1 / 2 \mathrm{~N}_{2}(\mathrm{~g})+1 / 2 \mathrm{O}_{2}(\mathrm{~g})$ at the same temperature is :-
Correct Option: 1
Solution:
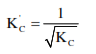
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
