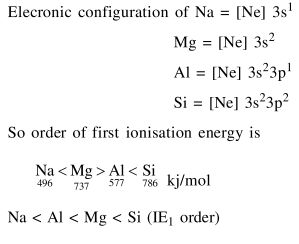
Question: The first ionization energy (in $\mathrm{kJ} / \mathrm{mol}$ ) of $\mathrm{Na}$, $\mathrm{Mg}, \mathrm{Al}$ and $\mathrm{Si}$ respectively, are :
$496,737,577,786$
$786,737,577,496$
$496,577,737,786$
$496,577,786,737$
Correct Option: 1
Solution: