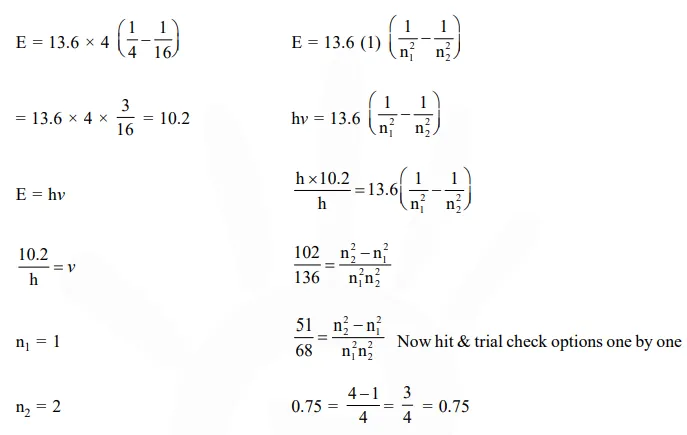Question: The frequency of light emitted for the transition $\mathrm{n}=4$ to $\mathrm{n}=2$ of $\mathrm{He}^{+}$is equal to the transition in $\mathrm{H}$ atom corresponding to which of the following
$\mathrm{n}=3$ to $\mathrm{n}=1$
$\mathrm{n}=2$ to $\mathrm{n}=1$
$\mathrm{n}=3$ to $\mathrm{n}=2$
$n=4$ to $n=3$
Correct Option: , 2
Solution: