Question: 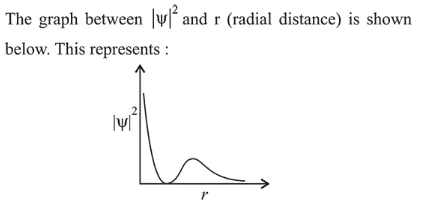
$3 s$ orbital
$2 s$ orbital
$1 s$ orbital
$2 p$ orbital
Correct Option: , 2
Solution:
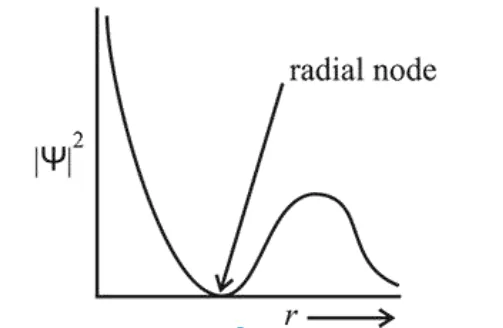
The given probability density curve is for $2 s$ orbitaı due to the presence of only one radial node. $1 s$ and $2 p$ orbital do not have any radial node and $3 s$ orbital has two radial nodes. Hence, option (2) is correct.