Question:
The increasing order of $\mathrm{p} K_{\mathrm{b}}$ for the following compounds will be:
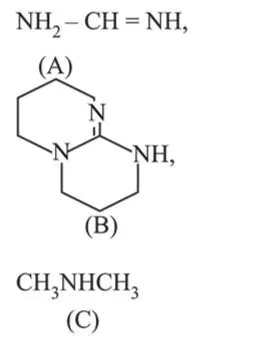
Correct Option: , 4
Solution:
Conjugate acid of guanadine(B) is resonance stabilised and have 2 resonance structure.
Similarly conjugate acid of (A) is also resonance stabilised and have one resonance structure. (C) does not exhibit resonance structure.
therefore the basic order is, $k_{b}:(B)>(A)>(C)$
$\therefore \mathrm{pk}_{\mathrm{b}}:(\mathrm{B})<(\mathrm{A})<(\mathrm{C})$
