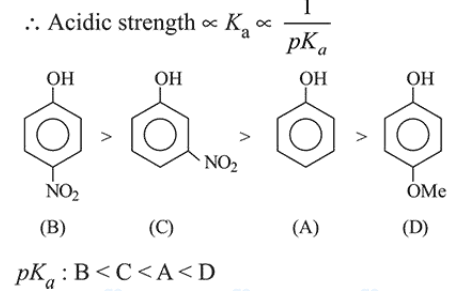
Question: The increasing order of the $\mathrm{p} K_{\mathrm{a}}$ values of the following compounds is:
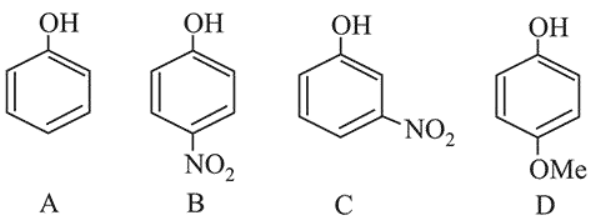
$\mathrm{C}<\mathrm{B}<\mathrm{A}<\mathrm{D}$
$\mathrm{B}<\mathrm{C}<\mathrm{D}<\mathrm{A}$
$\mathrm{D}<\mathrm{A}<\mathrm{C}<\mathrm{B}$
$\mathrm{B}<\mathrm{C}<\mathrm{A}<\mathrm{D}$
Correct Option: , 4
Solution:
Electron withdrawing substituents increase the acidic strength while electron releasing groups decrease the acidic strength.