Question:
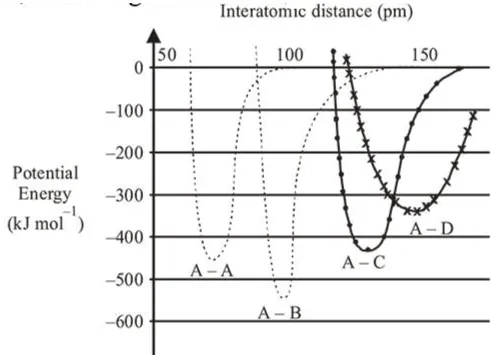
The intermolecular potential energy for the molecules $A$, $B, C$ and $D$ given below suggests that :
Correct Option: , 4
Solution:
A-B bond has highest intermolecular potential energy among the given molecules. Hence, it is strongest bond and has maximum bond enthalpy.
