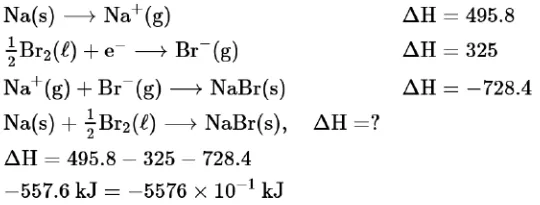Question:
The ionization enthalpy of $\mathrm{Na}^{+}$formation from $\mathrm{Na}_{(g)}$ is $495.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$, while the electron gain enthalpy of Br is $-325.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$. Given the lattice enthalpy of $\mathrm{NaBr}$ is $-728.4 \mathrm{~kJ} \mathrm{~mol}^{-1}$. The energy for the formation of $\mathrm{NaBr}$ ionic solid is (-)____________ $\times 10^{-1} \mathrm{~kJ} \mathrm{~mol}^{-1}$
Solution:
$(\mathbf{5 5 7 6})$