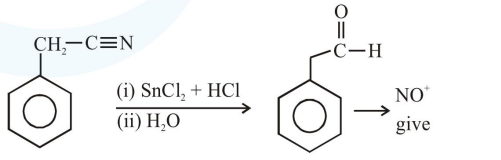
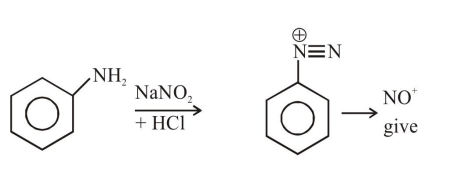
Question: The Kjeldahl method of Nitrogen estimation fails for which of the following reaction products ?
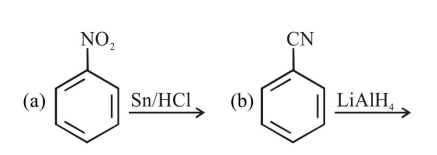
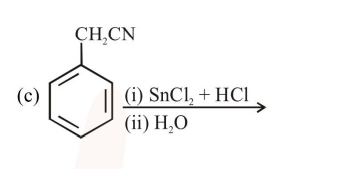
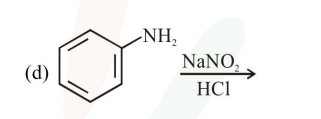
a and d
c and $\mathrm{d}$
$\mathrm{a}, \mathrm{c}$ and $\mathrm{d}$
$b$ and $c$
Correct Option: 2,
Solution:
Kjeldahl method is used for $\mathrm{N}$ estimation But not given by 'Diazo' compounds