Question:
The molecular geometry of $\mathrm{SF}_{6}$ is octahedral. What is the geometry of $\mathrm{SF}_{4}$ (including lone pair(s) of electrons, if any) ?
Correct Option: 1
Solution:
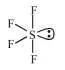
$4 \sigma$ bonds $+1$ lone pair
$\therefore$ Shape (including lone pair of electrons) is
Trigonal bipyramidal
