Question:
The $\mathrm{P}-\mathrm{V}$ diagram of a diatomic ideal gas system going under cyclic process as shown in figure. The work done during an adiabatic process $\mathrm{CD}$ is (use $\gamma=1.4)$
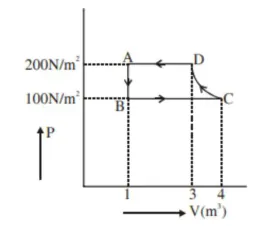
Correct Option: 1
Solution:
(1)
Adiabatic process is from $\mathrm{C}$ to $\mathrm{D}$
$\mathrm{WD}=\frac{\mathrm{P}_{2} \mathrm{~V}_{2}-\mathrm{P}_{1} \mathrm{~V}_{1}}{1-\gamma}$
$=\frac{P_{\mathrm{D}} V_{\mathrm{D}}-\mathrm{P}_{\mathrm{C}} \mathrm{V}_{\mathrm{C}}}{1-\gamma}$
$=\frac{200(3)-(100)(4)}{1-1.4}$
$=-500 \mathrm{~J} \quad$ Ans.(a)
