Question:
The reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
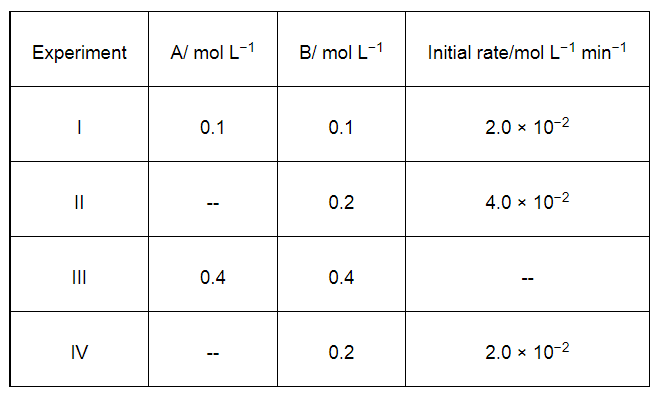
Solution:
The given reaction is of the first order with respect to A and of zero order with respect to B.
Therefore, the rate of the reaction is given by,
Rate = k [A]1 [B]0
⇒ Rate = k [A]
From experiment I, we obtain
2.0 × 10−2 mol L−1 min−1 = k (0.1 mol L−1)
⇒ k = 0.2 min−1
From experiment II, we obtain
4.0 × 10−2 mol L−1 min−1 = 0.2 min−1 [A]
⇒ [A] = 0.2 mol L−1
From experiment III, we obtain
Rate = 0.2 min−1 × 0.4 mol L−1
= 0.08 mol L−1 min−1
From experiment IV, we obtain
2.0 × 10−2 mol L−1 min−1 = 0.2 min−1 [A]
⇒ [A] = 0.1 mol L−1
