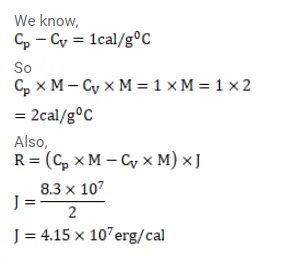Question:
The specific heat capacities of hydrogen at constant volume and at constant pressure are $2.4 \mathrm{cal} / \mathrm{g}-{ }^{\circ} \mathrm{C}$ and $3.4 \mathrm{cal} / \mathrm{g}-{ }^{\circ} \mathrm{C}$ respectively, The molecular weight of hydrogen is $2 \mathrm{~g} / \mathrm{mol}$ and the gas constant $R$ $=8.3 \times 10^{7} \mathrm{erg} / \mathrm{mol}{ }^{\circ} \mathrm{c}$. Calculate the value of $\mathrm{J}$.
Solution: