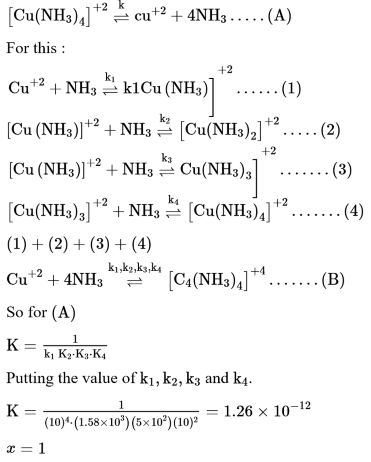
The stepwise formation of $\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right]^{2+}\right.$ is given below:
$\mathrm{Cu}^{2+}+\mathrm{NH}_{3} \stackrel{\mathrm{K}_{1}}{\Longrightarrow}\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$$\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}+\mathrm{NH}_{3} \stackrel{\mathrm{K}_{2}}{\Longrightarrow}\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}$$\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{2}\right]^{2+}+\mathrm{NH}_{3} \stackrel{\mathrm{k}_{3}}{=}\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}$$\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{3}\right]^{2+}+\mathrm{NH}_{3} \stackrel{\mathrm{k}_{4}}{\Longrightarrow}\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$The value of stability constants $K_{1}, K_{2}, K_{3}$ and $K_{4}$ are $10^{4}, 1.58 \times 10^{2}, 5 \times 10^{2}$ and $10^{2}$respectively. The overall equilibrium constants for dissociation of $\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$ is $\mathrm{x} \times 10^{-12}$. The value of $x$ is________ . (Rounded off to the nearest integer)
(1)