Question:
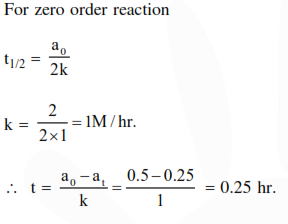
The time for half life period of a certain reaction $\mathrm{A} \longrightarrow$ Products is 1 hour, when the initial concentration of the reactant 'A' is $2.0 \mathrm{~mol} \mathrm{~L}-1$, How much time does it take for its concentration to come from $0.50$ to $0.25 \mathrm{~mol} \mathrm{} \mathrm{L}^{-1}$ if it is a zero order reaction?
Correct Option: , 4
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
