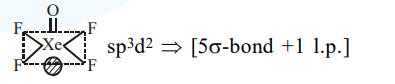Question: The type of hybridisation and number of lone pair(s) of electrons of $\mathrm{Xe}$ in $\mathrm{XeOF}_{4}$, respectively, are :
$\operatorname{sp}^{3} d$ and 1
$s p^{3} d$ and 2
$\mathrm{sp}^{3} \mathrm{~d}^{2}$ and 1
$s p^{3} d^{2}$ and 2
Correct Option: , 3
Solution: