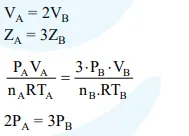Question: The volume of gas A is twice than that of gas B. The compressibility factor of gas A is thrice than that of gas B at same temperature. The pressures of the gases for equal number of moles are :
$2 \mathrm{P}_{\mathrm{A}}=3 \mathrm{P}_{\mathrm{B}}$
$\mathrm{P}_{\mathrm{A}}=3 \mathrm{P}_{\mathrm{B}}$
$\mathrm{P}_{\mathrm{A}}=2 \mathrm{P}_{\mathrm{B}}$
$3 \mathrm{P}_{\mathrm{A}}=2 \mathrm{P}_{\mathrm{B}}$
Correct Option: 1
Solution: