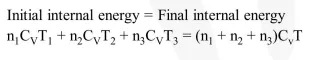Question:
Three perfect gases at absolute temperatures $\mathrm{T}_{1}, \mathrm{~T}_{2}$ and $\mathrm{T}_{3}$ are mixed. The masses of molecules are $\mathrm{m}_{1}, \mathrm{~m}_{2}$, and $\mathrm{m}_{3}$ and the number of molecules are $\mathrm{n}_{1}, \mathrm{n}_{2}$ and $\mathrm{n}_{3}$ respectively. Assuming no loss of energy, then final temperature of the mixture is :-
Correct Option: , 4
Solution: