Question:
To find out the concentration of $\mathrm{SO}_{2}$ in the air (in parts per million, i.e., ppm), the data was collected for 30 localities in a certain city and is presented below.
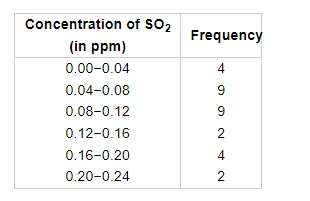
Find the mean of concentration of $\mathrm{SO}_{2}$ in the air.
Solution:
Let the assumed mean A = 0.1 and h = 0.04.
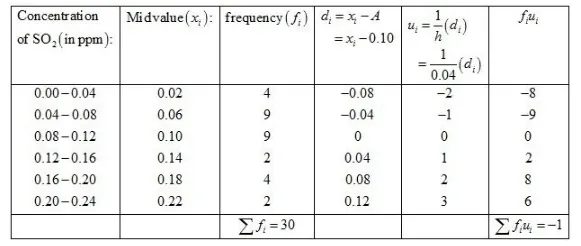
We know that mean, $\bar{X}=A+h\left(\frac{1}{N} \sum f_{i} u_{i}\right)$
Now, we have $N=\sum f_{i}=30, \sum f_{i} u_{i}=-1, h=0.04$ and $A=0.10$.
Putting the values in the above formula, we have
$\bar{X}=A+h\left(\frac{1}{N} \sum f_{i} u_{i}\right)$
$=0.10+0.04\left[\frac{1}{30} \times(-1)\right]$
$=0.10-\frac{0.04}{30}$
$=0.10-0.001$
$=0.099$
Hence, the mean concentration of $\mathrm{SO}_{2}$ in the air is $0.099 \mathrm{ppm}$.
