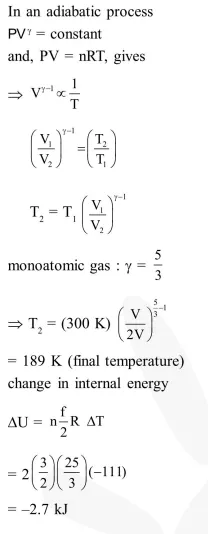
Question:
Two moles of an ideal monoatomic gas occupies a volume $\mathrm{V}$ at $27^{\circ} \mathrm{C}$. The gas expands adiabatically to a volume $2 \mathrm{~V}$. Calculate (a) the final temperature of the gas and (b) change in its internal energy.
Correct Option: , 2
Solution: